(Reaction Biology 한국독점대리점_에디스젠_edithgen@daum.net) SPR Binding assay service (Reaction Biology 한국독점_에디스젠)
안녕하세요
Reaction Biology 한국독점대리점_에디스젠입니다
항상 최선을 다하겠습니다
감사합니다
에디스젠 (Reaction Biology 한국독점대리점) 배상
Surface Plasmon Resonance Assay Services for Drug Discovery
Surface Plasmon Resonance (SPR) is a highly sensitive technique for accurate analysis of the interactions of two biomolecules with respect to binding kinetics and affinity as well as binding specificity.
To perform SPR assays, Reaction Biology is equipped with two state-of-the-art Biacore 8K and two 8K+ instruments with high-throughput compound screening capability and extraordinary detection sensitivity. We perform high-throughput fragment screening, kinetics and affinity determination, binding specificity profiling and antibody characterization, etc.
Reaction Biology provides project-tailored solutions to assure the highest chance of success in biomolecular interaction research using SPR services by using a broad biophysics knowledge base and excellent instrument coverage.
- The SPR binding assay is suitable to advance any analyte including fragments, antibodies, peptides, nucleic acids against any target class including enzymes and non-active proteins
- SARS-CoV-2 S protein and ACE2 receptor binding assay is available here
- Deliverables: association rate constant kon, dissociation rate constant koff, binding affinity KD
Our experts have extensive expertise in analyzing and resolving surface plasmon resonance challenges with ‘difficult to test’ proteins. The assay is carried out at Malvern, Pennsylvania, in the United States and performed on a first-come, first-serve basis.
SPR Binding Assay Principle
SPR analysis is an optical method measuring changes in the mass of biomolecules immobilized on a metal film. Upon binding an analyte, the refractive index of the metal film changes, resulting in a changed reflection angle of light (the surface plasmon resonance phenomenon). SPR binding assays are widely used in drug discovery, from fragment screening through analyte ranking and in-depth kinetic characterization.
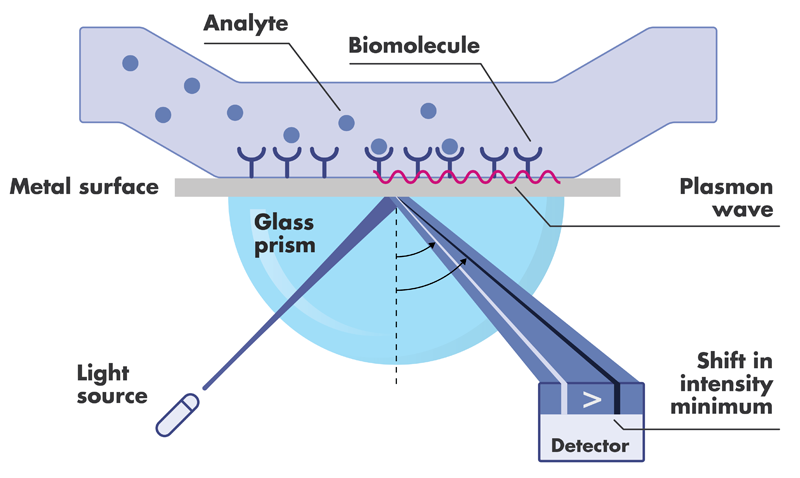
List of Surface Plasmon Resonance Assays
| ACE2 | ACE2 | Angiotensin I converting enzyme 2 | Data sheet |
| B-cell lymphoma-2 | BCL2 | BCL-2 | Data sheet |
| Bcl-2-like protein 1 isoform L | BCL2L1 | BCL-XL | Data sheet |
| Bcl-2-like protein 2 | BCL2L2 | BCLW, KIAA0271 | Data sheet |
| BRD2-1 | BRD2 | O27.1.1, Really interesting new gene 3 protein | Data sheet |
| BRD2-2 | BRD2 | O27.1.1, Really interesting new gene 3 protein | Data sheet |
| BRD3-1 | BRD3 | RING3-like protein | Data sheet |
| BRD3-2 | BRD3 | RING3-like protein | Data sheet |
| BRD4-1 | BRD4 | Bromodomain containing 4, domain 1 | Data sheet |
| BRD4-2 | BRD4 | Bromodomain containing 4, domain 2 | Data sheet |
Additional Information for SPR Binding Assays
- Kinetics analysis
- Example: Co-factor anaylsis
- Example: Binding affinity
- Screening Details
- Assay Development
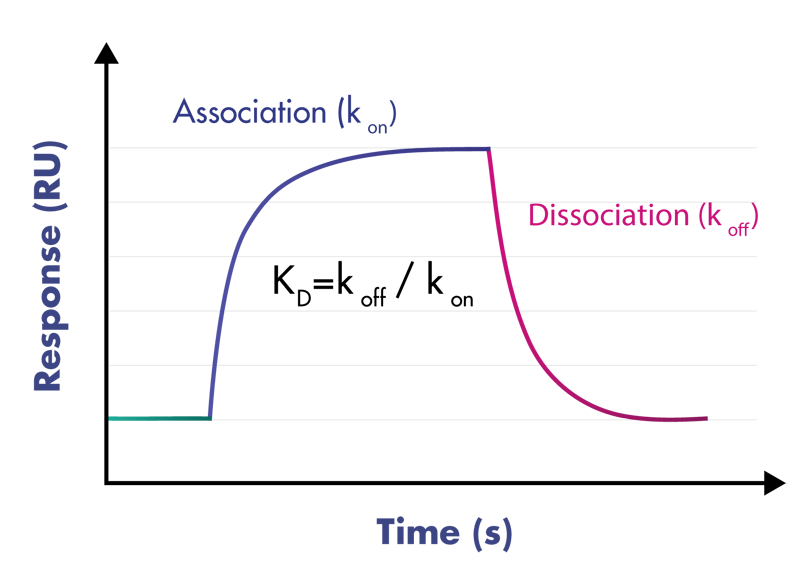
Kinetic profile of an analyte-target binding reaction.
SPR detects changes in the refractive index at the surface of a sensor chip as a result of molecular mass changes of a target upon binding of the analyte. The target is immobilized to the surface of the sensor chip. During the association phase, the analyte flows over the surface, and binding to the target is monitored. The flow then switches to the running buffer, and the dissociation of the analyte from the target is monitored.
Application
| Target is not an enzyme or unknown substrate | SPR measures the direct binding between analyte and target which is why the target does not need to be an enzyme and no substrate is needed. |
| Co-factor/competition studies | The impact of various co-factors on analyte-target interaction can be tested. |
| Fragment-based screening | Low molecular mass fragment compounds (100–300 Da) tend to demonstrate low binding affinity; thus, the compounds require screening at a high concentration. This is better tolerated in the SPR platform than in many biochemical assays. |
| Antibody screening/ characterization |
SPR can be used for antibody affinity determination, determination of kinetic parameters, epitope mapping, binding specificity, and cross-reactivity. |
| SAR studies | The kinetics of drug binding and unbinding, especially the residence time, play a crucial role in a drug’s in vivo efficacy. SPR can rank the kinetic selectivity of drug analogs for the selection of the best drug candidates. |
| High-information content | Combining kinetic information with affinity and potency data early in the drug discovery process ensures that promising compounds are not being discarded. |
| Elimination of promiscuous binders | Promiscuous binders, which appear as false positives in biochemical inhibitor assays, can be identified by SPR when used as the secondary screening technology. |
| Plasma protein binding | SPR-based assays are sensitive high-throughout options to accurately measure plasma protein binding of analytes. |




